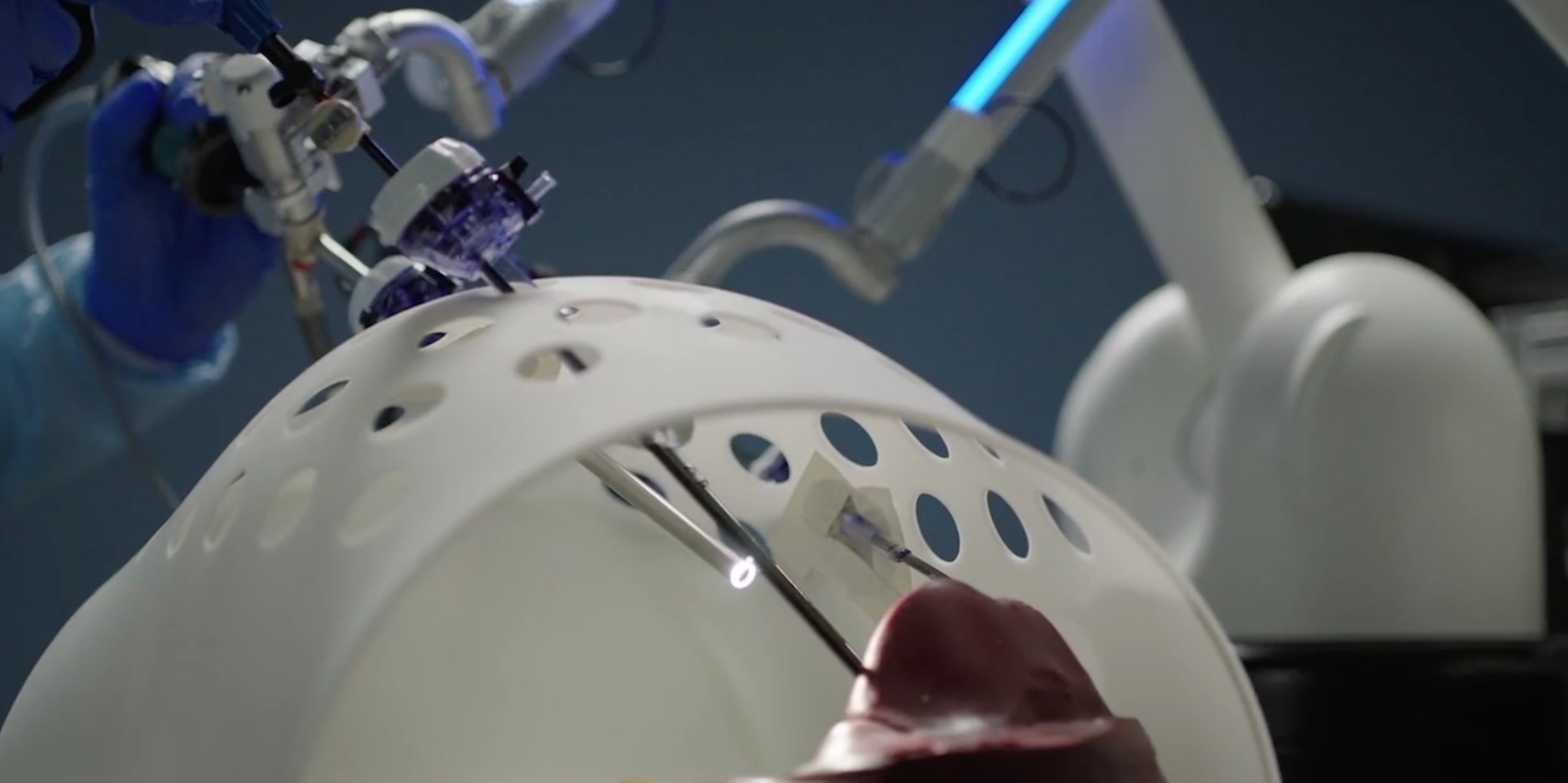
Less than 3 years ago a prototype of the ISIR’s surgical cockpit was was transferred from ISIR to Moon Surgical, thanks to the help of SUMMIT – Maison des Modélisations Ingénieries et Technologies. Moon Surgical’s team worked without rest to transform the lab prototype to an industrialized product that just got FDA-approved.
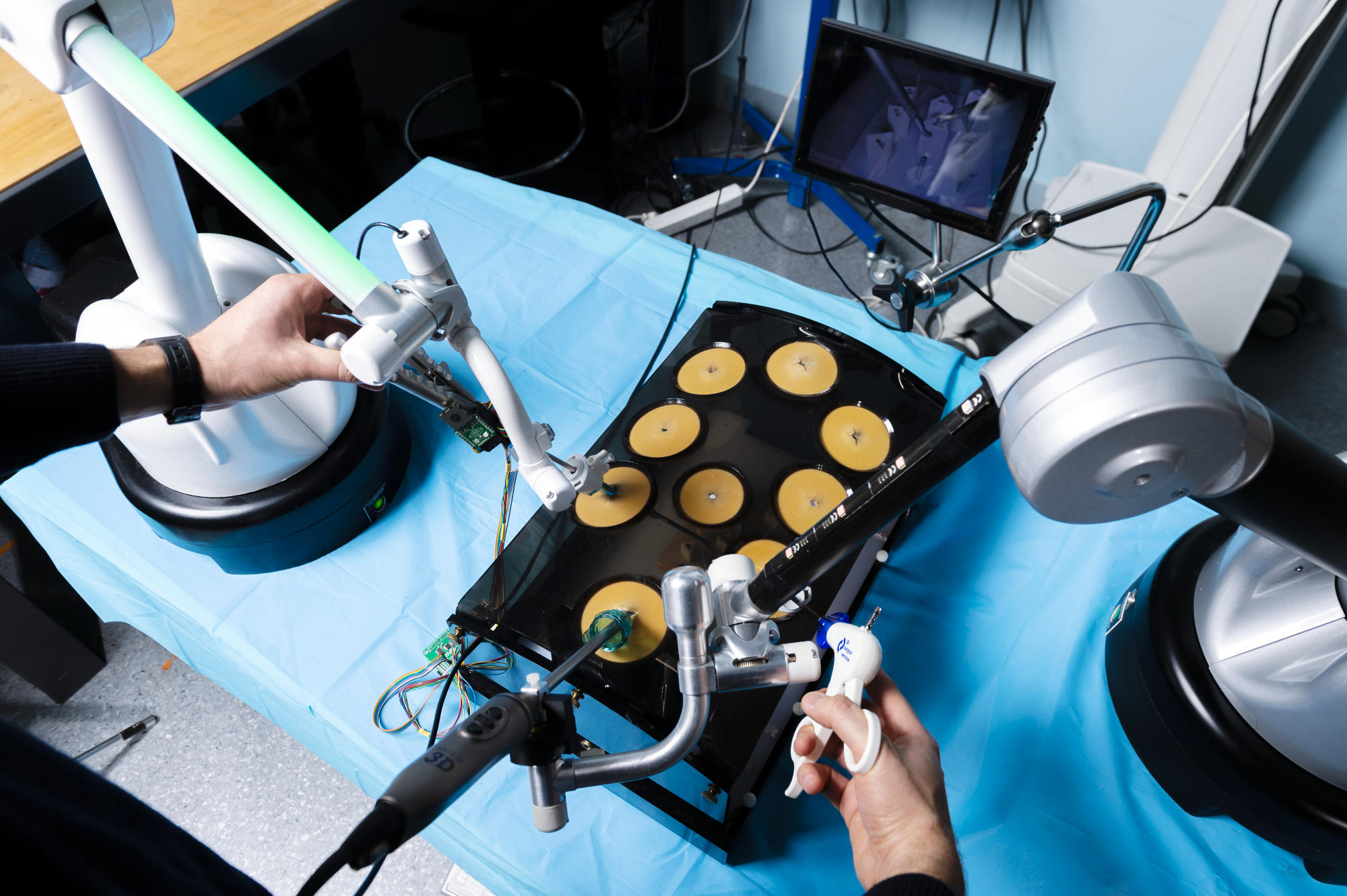
Maestro (it’s new name), was designed within the CAMI Labex context with Brice Gayet, surgeon and pioneer in laparoscopy, as a medical leader, and Guillaume Morel and Marie-Aude Vitrani as scientific leaders.

Moon Surgical, a French-American pioneer in accessible surgical innovation, has just announced the United States Food and Drug Administration (FDA) clearance for its unique Maestro surgical robotics system, designed to augment the surgeon at the bedside in minimally invasive soft tissue surgical procedures.
The Maestro platform becomes one of the very few surgical robotics systems authorized on the market in the United States for laparoscopic surgery. This success significantly changes the future accessibility to surgical innovation.
More information with the press release
Link to the article “Robots in the blocks”